

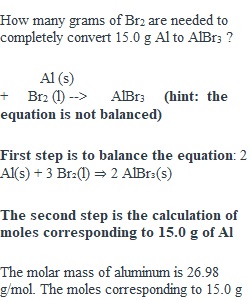
Q Learning Objectives • calculate a mass/mass stoichiometry problem using conversion factors required for the operation • Use all units (including the names of substances involved) when solving stoichiometry problems • Report your answer according to the rules of arithmetic with measured values Directions Solve the following problem showing all of your work and including all units. You can solve the problem in several steps or as a tandem conversion problem. How many grams of Br2 are needed to completely convert 15.0 g Al to AlBr3 ? Al (s) + Br2 (l) --> AlBr3 (hint: the equation is not balanced) Submission Instructions Click Start Assignment and type your answers in the Rich Content Editor here in Canvas. If you prefer you can submit your answer as a pdf or Word document upload. ________________________________________ Grading and Feedback I am grading your response on the accuracy of your answer and your proper use of the significant figure rules. I am also grading your answer on your correct use of conversion ratios and units.. You can view the Rubric below. The assignment feedback will be provided and points will be awarded within 3 days of the final submission date. Rubric ________________________________________ Technical Support Need help using Canvas Assignments? If so, please review the following Canvas Guide pages: • How do I submit an online assignment? (Links to an external site.) • How do I upload a file as an assignment submission in Canvas? (Links to an external site.) • How do I submit a text entry assignment? (Links to an external site.) • How do I enter a URL as an assignment submission? (Links to an external site.) Rubric Stoichiometry discussion Stoichiometry discussion Criteria Ratings Pts This criterion is linked to a Learning OutcomeProper approach and use of units Did you set up your problem correctly and did you use units in your ratios. 5 to >0.0 pts Full Marks Use of units and correct ratios. 0 pts No Marks No work shown 5 pts This criterion is linked to a Learning OutcomeSolve problem correctly Is your problem solved correctly and did you report your answer with the proper number of sig figs and/or correct degree of precision 15 pts Full Marks Correct answer and proper solution 9 pts Correct solution Correct solution but answer reported incorrectly 0 pts No Marks Incorrect solution 15 pts Total Points: 20 PreviousNext
View Related Questions